Preeclampsia Diagnostics Market By Diagnostic Test Type (Blood tests, Urine tests, Ultrasound, Doppler ultrasound, Angiogenic factor testing); By End-User (Hospitals, Diagnostic laboratories, Clinics, Prenatal care centers); By Technology (Immunoassays, Molecular diagnostics, Point-of-care testing, Imaging technologies); By Mode of Testing (Laboratory-based testing, Point-of-care testing); By Region (North America, Europe, Asia Pacific, Latin America, Middle East & Africa) - Global Market Analysis, Trends, Opportunity and Forecast, 2022-2032
Preeclampsia Diagnostics Market Insights
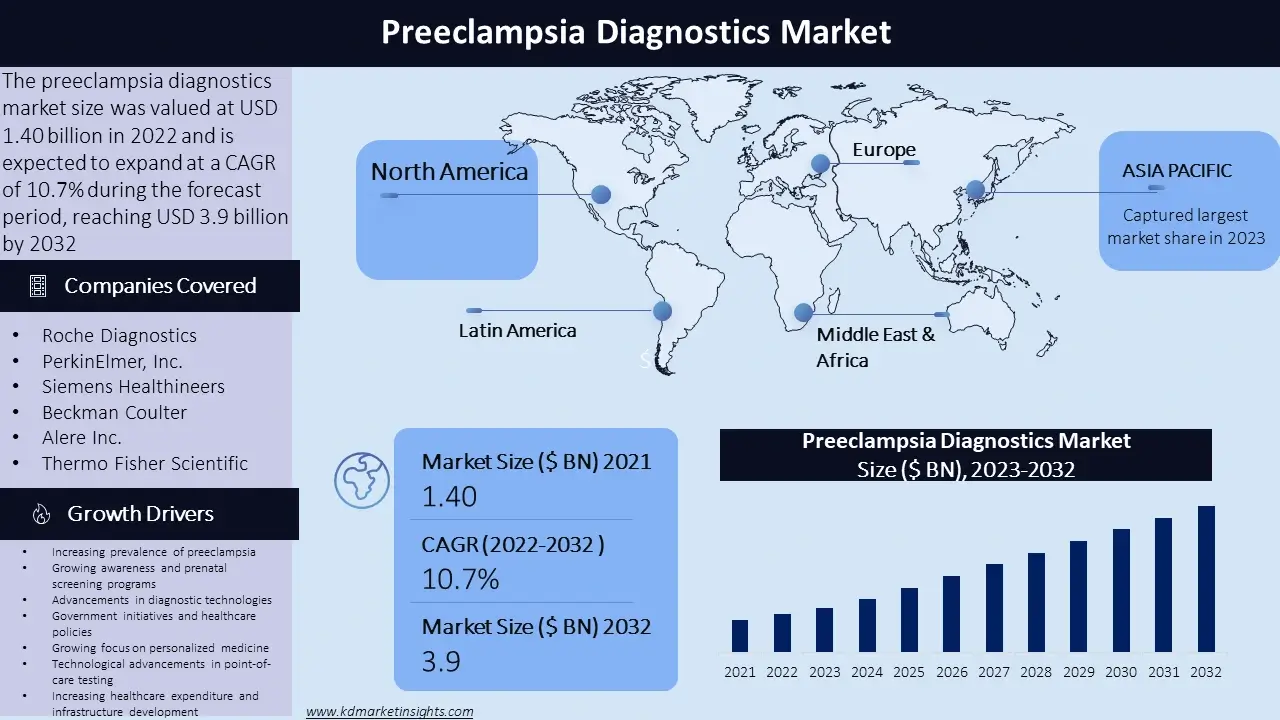
The preeclampsia diagnostics market size was valued at USD 1.4 billion in 2022 and is expected to expand at a CAGR of 10.7% during the forecast period, reaching USD 3.9 billion by 2032. The preeclampsia diagnostics market refers to the market for individual tests and tools used to detect and diagnose preeclampsia, a potentially serious condition that can do during pregnancy. Preeclampsia is characterized by high blood pressure and damage to organs, most commonly affecting the liver and kidney.
Preeclampsia Diagnostic Market: Report Scope |
|
|
Base Year Market Size |
2022 |
|
Forecast Year Market Size |
2023-2032 |
|
CAGR Value |
10.7% |
|
Segmentation |
|
|
Challenges |
|
|
Growth Drivers |
|
Preeclampsia Diagnostics Market Driving Factors
Some Driving factors of Preeclampsia Diagnostics Market are:
Increasing prevalence of preeclampsia: The rising incidence of preeclampsia worldwide is a significant driver for the diagnostics market. Factors similar as maternal age, obesity, multiple pregnancies, and underlying health conditions contribute to the adding prevalence.
Growing awareness and prenatal screening programs: Awareness about the risks and complications associated with preeclampsia has led to the implementation of parental screening programs. These programs aim to identify high risk pregnancy and enable early detection and management of preeclampsia, thereby driving the demand for diagnostic tests.
Advancements in diagnostic technologies: The accuracy and efficiency of preeclampsia opinion have improved with the development of advanced diagnostic technologies, such as biomarker-based testing, imaging techniques, and non-invasive monitoring methods. The need has increased as a result of these developments, and healthcare practitioners now have better tools for early detection and intervention.
Government initiatives and healthcare policies: Preeclampsia diagnostics are in more demand due to government initiatives encouraging maternal and foetal health as well as healthcare policies emphasising routine prenatal care. These initiatives seek to improve pregnancy problems and reduce the burden of consequences caused by preeclampsia.
Growing focus on personalized medicine: The demand for preeclampsia diagnostics has increased with the move towards individual care and personalised medicine. Individual tests that enable risk stratification and tailored management based on the patient condition and characteristics of the case are highly demanded.
Technological advancements in point-of-care testing: Point-of-care testing devices and kit have made quick and easy preeclampsia diagnosis possible. These portable and user-friendly devices produce immediate results, allowing for prompt responses and reduce the need for laboratory testing.
Increasing healthcare expenditure and infrastructure development: The expansion of the preeclampsia diagnostics market has been influenced by the growth of healthcare spending, especially in emerging economies. Increased testing rates and the early detection of preeclampsia cases are results of improved healthcare infrastructure and access to individual installations.
Preeclampsia Diagnostics Market Challenges
The preeclampsia diagnostics market faces several challenges:
Lack of specific biomarkers: The lack of specific indicators for preeclampsia makes it difficult to form a reliable assessment. The methods used by current individual tests, which combine clinical symptoms, laboratory parameters, and symptoms, may not provide definitive results.
Variability in presentation: Preeclampsia can present with a wide variety of symptoms and severity level, which complicates diagnosis. Early detection and accurate diagnosis are made more difficult by the variability in clinical presentation.
Limited access to diagnostic tools: In some places, especially in low-resource environments, preeclampsia opinion access to advanced diagnostic tools and technology may be limited. This prevents prompt and accurate diagnosis leading to potential risk for both the mother and the foetus.
Cost implications: Preeclampsia tests can be expensive individually, which restricts their accessibility, especially in settings with limited resources. The high price may make it difficult for many people to give up and use of diagnostics tools.
Interpretation and standardization: Preeclampsia test findings interpretation might be subjective, which causes variation among medical professionals. To ensure consistent and accuracy of opinion, individual criteria and interpretation guidelines must be standardised.
Limited awareness and education: Healthcare professionals and pregnant women can delay their opinions due to a lack of awareness and education about preeclampsia. For early identification and efficient management, improving awareness and education about the disease and its effective management are crucial.
Ethical considerations: Invasive treatments and tests are used in preeclampsia diagnosis, which may create ethical concerns for pregnant women. In the field of preeclampsia diagnostics, it might be difficult to strike a balance between the need for a correct assessment and the ethical consideration and potential risk.
Preeclampsia Diagnostics Market Regional Synopsis
Regional synopsis of the preeclampsia diagnostics market:
North America: The need for preeclampsia diagnostics in North America is well established and is driven by advanced healthcare infrastructure, activities in research and development, and high awareness level. The region places a lot of emphasis on the early diagnosis and treatment of preeclampsia, which has led to the adoption of advanced diagnostic technologies and screening programmes.
Europe: Preeclampsia diagnostics are growing market in Europe, with countries ilike the United Kingdom, Germany, and France taking the lead in terms of research and development, healthcare infrastructure, and prenatal screening programmes. The region emphasizes accurate and timely diagnosis, resulting in the widespread use of diagnostic tests and tools.
Asia-Pacific: The Asia- Pacific region has a growing market for preeclampsia diagnostics due to the increasing prevalence of the condition and improving healthcare infrastructure. Countries like China, India, and Japan have witnessed a rise in awareness about preeclampsia, leading to the adoption of diagnostic tests for early detection and management.
Latin America: Latin America has a developing market for preeclampsia diagnostics, with countries similar as Brazil, Mexico, and Argentina plays a significant role. The region has been making efforts to improve parental care and reduce motherly mortality rates, leading to increased focus on preeclampsia opinion and webbing programs.
Middle East and Africa: The Middle East and Africa region have a growing awareness about preeclampsia and its complications, leading to an adding demand for individual tools and tests. Countries similar as Saudi Arabia, South Africa, and UAE are investing in healthcare structure and enforcing programs to enhance preeclampsia opinion.
Preeclampsia Diagnostics Market Segmentation
Some segmentation of preeclampsia diagnostics market are:
By Diagnostic Test Type
- Blood tests
- Urine tests
- Ultrasound
- Doppler ultrasound
- Angiogenic factor testing
By End-User
- Hospitals
- Diagnostic laboratories
- Clinics
- Prenatal care centers
By Technology
- Immunoassays
- Molecular diagnostics
- Point-of-care testing
- Imaging technologies
By Mode of Testing
- Laboratory-based testing
- Point-of-care testing
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
- Rest of the World.
Preeclampsia Diagnostics Market Key Players
Some key players in the preeclampsia diagnostics market are:
- Roche Diagnostics
- PerkinElmer, Inc.
- Siemens Healthineers
- Beckman Coulter
- Alere Inc.
- Thermo Fisher Scientific
- Sera Prognostics
- Metabolomic Diagnostics
- BioMérieux
- Agilent Technologies

Need Customized Report for Your Business ?
Utilize the Power of Customized Research Aligned with Your Business Goals
Request for Customized Report- Quick Contact -
- ISO Certified Logo -

















