Hereditary Angioedema Therapeutics Market By Distribution Channel (Hospital pharmacies, Retail pharmacies, Online pharmacies); By End users (Hospitals, Clinics, Home Care Settings); By Route of Administration (Intravenous, Subcutaneous, Oral); By Treatment Type (C1 esterase inhibitor, Kallikrein inhibitors, Bradykinin B2 receptor antagonists); By Geography (North America, Europe, Asia-Pacific, Latin America, Middle East & Africa); By Region (North America, Europe, Asia Pacific, Latin America, Middle East & Africa) - Global Market Analysis, Trends, Opportunity and Forecast, 2022-2032
Hereditary Angioedema Therapeutics Insights
Hereditary Angioedema (HAE) is a rare genetic disease characterized by recurrent episodes of swelling in various parts of the body, including the face, extremities, genitals, and gastrointestinal system. It is caused by a deficiency or dysfunction of the C1 inhibitor, which may be a protein that helps regulate the body's immune reaction and control inflammation. There are three sorts of HAE:
- Type I HAE: This is often the foremost common type caused by a deficiency of a C1 inhibitor.
- Type II HAE: Caused by a dysfunctional C1 inhibitor.
- Type III HAE: A rare sort of HAE that primarily affects women and is related to normal C1 inhibitor levels.
Symptoms of HAE typically include episodes of swelling which will last for several days, also as abdominal pain, nausea, and vomiting. Swelling within the airways also can occur and cause life-threatening complications like difficulty breathing. It is often diagnosed through blood tests to live C1 inhibitor levels or genetic testing. Treatment may involve medications to stop or treat attacks, like plasma-derived or recombinant C1 inhibitor replacement therapy, bradykinin receptor antagonists, or kallikrein inhibitors. Patients can also be advised to avoid triggers like stress, infections, or certain medications.
Hereditary Angioedema Therapeutics Market Share and Size
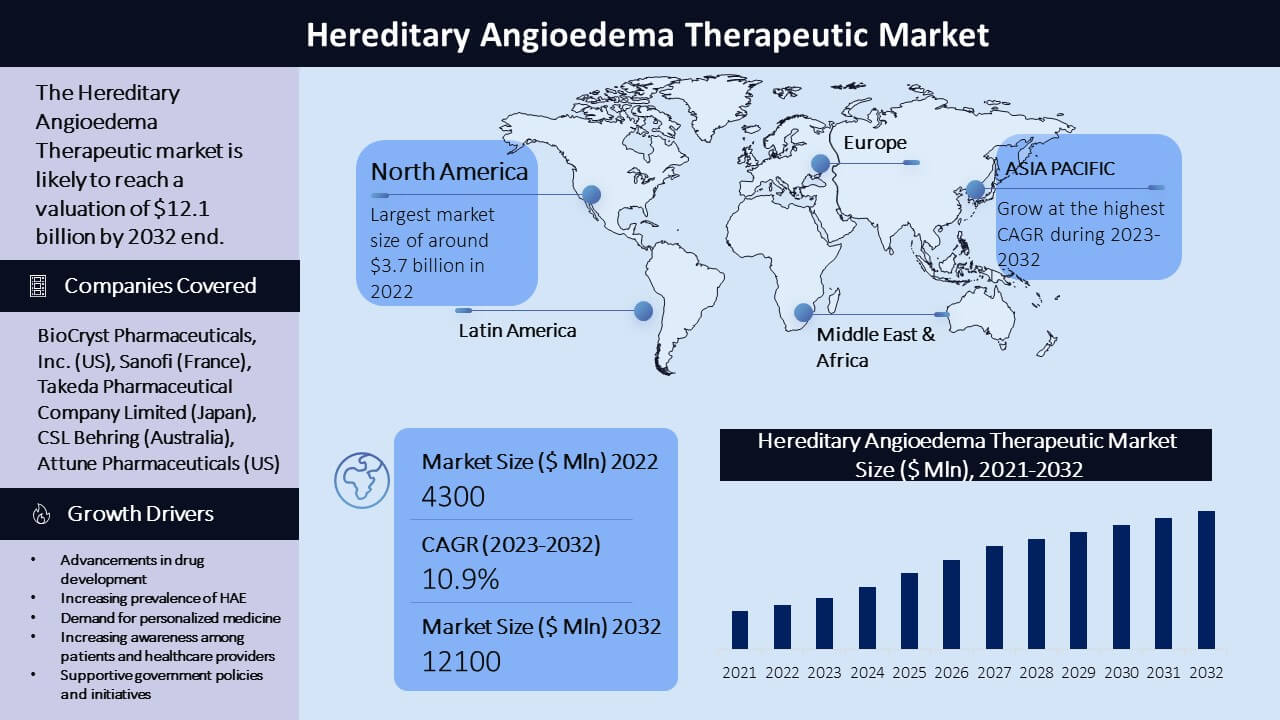
The Hereditary Angioedema (HAE) therapeutics market is driven by the increasing prevalence of HAE worldwide, also because of the development of the latest and simpler treatments. The global Hereditary Angioedema (HAE) therapeutics market was valued at approximately USD 4.3 billion in 2022 and is projected to succeed in USD 12.1 billion by 2032, with a compound annual rate of growth (CAGR) of 10.9% during the forecast period (2023-2032). Several companies currently offer HAE treatments, including Shire (part of Takeda drug company Limited), CSL Behring, Pharming Group NV, and BioCryst Pharmaceuticals. These companies offer a variety of therapies, including plasma-derived and recombinant C1 inhibitor replacement therapy, bradykinin receptor antagonists, and kallikrein inhibitors.
Hereditary Angioedema Therapeutics Market Key Driving Factors
There are several driving factors contributing to the growth of the Hereditary Angioedema (HAE) therapeutics market:
- Increasing prevalence of HAE: HAE is a rare genetic disease, but its prevalence is increasing worldwide. As more people are diagnosed with HAE, the demand for effective treatments is additionally increasing.
- Advancements in drug development: Over the past few years, several new and simpler drugs are developed to treat HAE. These drugs have shown significant improvements in reducing the frequency and severity of HAE attacks, which is driving market growth.
- Growing demand for personalized medicine: With advances in genetic testing and personalized medicine, healthcare providers are ready to better understand the underlying causes of HAE in individual patients leading to the development of more targeted and effective treatments.
- Increasing awareness among patients and healthcare providers: As more information becomes available about HAE, patients and healthcare providers are getting more conscious of the condition and therefore the need for effective treatments.
- Supportive government policies and initiatives: Governments around the world are implementing policies and initiatives to support the event and availability of rare disease treatments, including those for HAE.
Hereditary Angioedema Therapeutics Market Key Trend & Development
There are several key trends and development in the field of the HAE therapeutics market:
- Novel therapies: Several novel therapies including subcutaneous and intravenous C1-inhibitor replacement therapies, bradykinin receptor antagonists, kallikrein inhibitors, and complement pathway modulators have shown promising leads to reduce the frequency and severity of HAE attacks.
- Biosimilars: With the approval of the primary C1-inhibitor biosimilar in Europe in 2017, the marketplace for biosimilar HAE therapeutics is predicted to grow which are cost-effective alternatives to the prevailing therapies, thereby increasing patient access to treatment.
- Gene therapy: Researchers are exploring the utilization of gene therapy to correct the mutation that causes HAE, potentially providing a long-term cure for the disease.
- Personalized medicine: Researchers are exploring the utilization of biomarkers to predict the onset of HAE attacks, and to tailor treatment to individual patients supported by their genetic and clinical profiles.
Hereditary Angioedema Therapeutics Market Segmentation:
The Hereditary Angioedema (HAE) therapeutics market can be segmented based on the following:
- By Distribution Channel
- Hospital pharmacies
- Retail pharmacies
- Online pharmacies
- By End users
- Hospitals
- Clinics
- Home Care Settings
- By Route Of Administration
- Intravenous
- Subcutaneous
- Oral
- By Treatment Type
- C1 esterase inhibitor
- Kallikrein inhibitors
- Bradykinin B2 receptor antagonists
- By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa
Hereditary Angioedema Therapeutics Market Regional Synopsis
With a big share of the worldwide market, North America is the largest marketplace for HAE therapeutics. By 2032, the market is predicted to be worth $3.7 billion, growing at a CAGR of 9.2% (2023-2032), with the USA serving as the region's largest market against an earlier estimation of worth $1.5 billion in 2022.
Europe is another important marketplace for HAE therapeutics, with Germany, France, and Italy having a high prevalence of the condition. The presence of major pharmaceutical companies within the region has an impression on the market. By 2032, the market is predicted to be worth $2.7 billion, growing at a CAGR of 8.4% (2023-2032) against an earlier estimation of worth $1.2 billion in 2022.
The HAE therapeutics market is predicted to expand significantly within the Asia-Pacific region thanks to rising public awareness of HAE and improved healthcare infrastructure. The region's primary markets are Japan, China, and India. With a CAGR of 9.8% (2023-2032), the market is predicted to succeed in $1.5 billion by 2032 against an earlier estimation of worth $574.4 million in 2022.
Although the marketplace for HAE therapeutics in Latin America is comparatively small, it's anticipated to expand significantly within the coming years thanks to increased public awareness of HAE and improved regional healthcare infrastructure. With a CAGR of 9.5% (2023-2032), the market is predicted to succeed in $461.0 million by 2032 against an earlier estimation of worth $186.0 million in 2022.
The Middle East and African marketplace for HAE therapeutics is relatively small, but it's expected to grow within the coming years because the region's healthcare infrastructure gets better and more people become conscious of HAE. By 2032, the market is predicted to be worth $355.1 million, growing at a CAGR of 8.3% (2023-2032) against an earlier estimation of worth $160.0 million in 2022.
Hereditary Angioedema Therapeutics Market Challenges:
Despite the growth prospects of the hereditary angioedema (HAE) therapeutics market, several challenges could impact the development and commercialization of HAE therapeutics:
- Limited pool of patients: The patient population is comparatively small because of its rarity. Due to this, pharmaceutical companies won't be ready to invest in the development of the latest treatments.
- Expensive therapy: The present therapies for HAE are expensive, which can make it harder for patients, especially in developing nations, to urge treatment.
- Safety issues: When used for an extended time or in high doses, HAE treatments can pose significant safety risks. as an example, there's a link between some treatments and an increased risk of blood clots, which might be fatal.
- Inadequate awareness: Healthcare providers lack awareness and comprehension of HAE, particularly in developing nations. As a result, diagnosis and treatment are going to be delayed, which can have a big impact on patient outcomes.
- Regulatory difficulties: The regulatory environment for HAE therapeutics is usually challenging, especially when it involves the planning of clinical tests and the endpoints they measure which may end in delays in drug development and approval impacting the commercial viability.
Hereditary Angioedema Therapeutics Market Key Players:
Several key players exist in the hereditary angioedema (HAE) therapeutics market. Some of the major players in the market include:
- Adverum Biotechnologies, Inc.: Adverum is a clinical-stage gene therapy company that develops gene-based therapies for rare diseases, including HAE. Its HAE drug candidate, ADVM-053, is an adeno-associated virus (AAV) vector-based gene therapy that delivers the human C1 esterase inhibitor gene to liver cells to supply long-term production of the protein.
- Attune Pharmaceuticals, Inc.: Attune is a biotechnology company focused on the event of novel therapeutics for HAE and other rare diseases. Its lead HAE drug candidate, ATN-249, is a recombinant C1 esterase inhibitor designed to supply long-acting prophylaxis and on-demand treatment for HAE.
- BioCryst Pharmaceuticals, Inc.: BioCryst is a biotechnology company that focuses on the invention, development, and commercialization of oral small molecule therapies for rare diseases, including HAE. Its HAE drug, Orladeyo (berotralstat), is the first oral therapy approved for the prevention of HAE attacks in adults and youngsters.
- CSL Behring LLC: CSL Behring’s HAE product, Berinert, is a plasma-derived C1 esterase inhibitor (C1-INH) approved for the treatment of acute attacks of HAE.
- Ionis Pharmaceuticals, Inc.: Ionis is a biopharmaceutical company that develops RNA-targeted therapies for HAE including TAKHZYRO (lanadelumab), which is a subcutaneously administered antibody designed to stop HAE attacks by inhibiting plasma kallikrein.
- Pharming Group N.V.: Pharming is a biopharmaceutical company that develops innovative protein replacement therapies for rare diseases, like its HAE drug, Ruconest (recombinant C1-INH).
- Sanofi: A global drug company that develops and markets a good range of therapeutics, including several HAE drugs including Cinryze (C1-INH), a plasma-derived C1 esterase inhibitor approved for the treatment of acute attacks and prophylaxis of HAE, and Icatibant, a subcutaneously administered bradykinin B2 receptor antagonist approved for the treatment of acute HAE attacks.
- Takeda drug company Limited (formerly Shire): Takeda is a multinational drug company that acquired Shire, a number one biotech company that developed several HAE therapies, including Firazyr (icatibant), Cinryze (C1-INH), and Takhzyro (lanadelumab-flyo).
Hereditary Angioedema Therapeutics Market: Report Scope |
|
|
Base Year |
2022 |
|
Base Year Market Size |
USD 4.3 billion |
|
Forecast Year |
2023-2032 |
|
Forecast Year Market Size |
USD 12.1 billion |
|
CAGR Value |
10.9% |
|
Segmentation |
|
|
Challenges |
|
|
Growth Drivers |
|

Need Customized Report for Your Business ?
Utilize the Power of Customized Research Aligned with Your Business Goals
Request for Customized Report- Quick Contact -
- ISO Certified Logo -