CAR T-Cell Therapy Market - Global Size, Share, Trends, Growth and Forecast Year ( 2022 – 2032 )
CAR T-Cell Therapy Market Dynamics
CAR T-Cell Therapy Market by Product Type (Axicabtagene Ciloleucel, Tisagenlecleucel, Brexucabtagene Autoleucel, Lisocabtagene Maraleucel, Idecabtagene Vicleucel, Others) Cancer Type (Leukemia, Lymphoma, Multiple Myeloma, Others) End-Users (Hospitals, Clinics, Research Institutes) and Geographic Regions (North America, Europe, Asia Pacific, Latin America, Middle East and Africa): Industry Trends and Global Forecasts, 2023-2032.
Market Size and Overview:
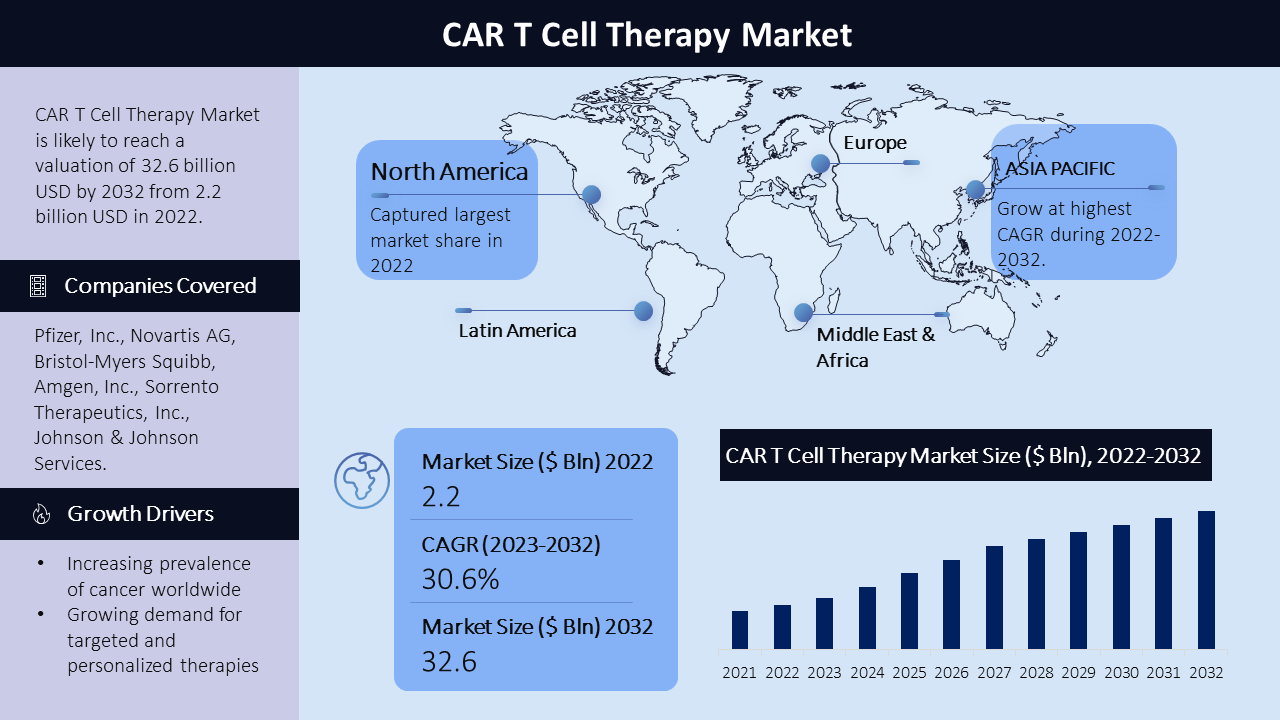
The CAR T cell therapy market has witnessed substantial growth, with a compound annual growth rate (CAGR) of 30.6% from 2023 to 2032, reaching a market value of $32.6 billion in 2032. This growth can be attributed to the increasing prevalence of cancer and the rising demand for targeted and personalized treatment options. CAR T cell therapy, a groundbreaking immunotherapy approach, has revolutionized the treatment landscape by leveraging a patient's own immune cells to target and eliminate cancer cells. The market comprises a diverse range of CAR T cell therapies targeting different types of cancer. Key players in the market continually strive to develop innovative therapies and expand their product pipelines to cater to the evolving needs of patients and healthcare providers. The market is highly competitive, with extensive research and development activities driving advancements in CAR T cell therapy.
|
CAR T Cell Therapy Market: Report Scope |
|
|
Base Year Market Size |
2022 |
|
Forecast Year Market Size |
2023-2032 |
|
CAGR Value |
30.6% |
|
Segmentation |
|
|
Challenges |
|
|
Growth Drivers |
|
Market Segmentation:
Product Type:
- Axicabtagene Ciloleucel
- Tisagenlecleucel
- Brexucabtagene Autoleucel
- Lisocabtagene Maraleucel
- Idecabtagene Vicleucel
- Others
Cancer Type:
- Leukemia
- Lymphoma
- Multiple Myeloma
- Others
End-Users:
- Hospitals
- Clinics
- Research Institutes
Geographic Regions:
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Leukemia: The leukemia segment holds the largest market share in the CAR T cell therapy market. The high prevalence of leukemia and the significant success of CAR T cell therapies in treating this cancer type have contributed to the segment's dominance. Continuous research and development efforts to enhance the efficacy and safety of CAR T cell therapies for leukemia are expected to drive further growth.
Hospitals: Hospitals represent a major end-user of CAR T cell therapy. With the increasing adoption of CAR T cell therapies and the need for specialized infrastructure and expertise to administer these treatments, hospitals play a crucial role in delivering CAR T cell therapy to patients. The establishment of dedicated CAR T cell therapy centers within hospitals further supports market growth.
Regional Analysis:
The regional analysis of the CAR T cell therapy market reveals distinct trends and dynamics across different regions. North America, particularly the United States, holds a significant market share, driven by advanced healthcare infrastructure, favorable reimbursement policies, and early adoption of innovative therapies. Europe, including countries like Germany, France, and the United Kingdom, is experiencing rapid growth, fueled by increasing investments in research and development and the approval of CAR T cell therapies by regulatory authorities. The Asia Pacific region shows immense growth potential, driven by a large patient population, rising awareness about CAR T cell therapy, and increasing healthcare expenditure. In Latin America, countries such as Brazil, Mexico, and Argentina are witnessing steady growth in the CAR T cell therapy market. Urban development, a rising middle class, and the demand for advanced cancer treatments are driving the market's expansion in the region. The Middle East and Africa region, including countries like the United Arab Emirates, Saudi Arabia, and South Africa, are also experiencing a developing market for CAR T cell therapy. The focus on urban development, and the growing emphasis on cancer treatment contribute to the market's growth in these regions.
Growth Drivers:
The CAR T cell therapy market is driven by several factors. Firstly, the increasing prevalence of cancer worldwide is a major driver for the adoption of CAR T cell therapy. CAR T cell therapies offer a promising treatment option for patients with relapsed or refractory cancers, who have limited treatment alternatives.
Furthermore, the growing demand for targeted and personalized therapies has fueled the uptake of CAR T cell therapy. By genetically modifying a patient's T cells to express chimeric antigen receptors (CARs), CAR T cell therapy enables precise targeting of cancer cells, minimizing damage to healthy tissues.
Moreover, advancements in CAR T cell therapy manufacturing and delivery processes have contributed to market growth. Streamlined manufacturing techniques have improved the scalability and cost-effectiveness of CAR T cell therapies, making them more accessible to patients.
Additionally, collaborations and strategic partnerships between pharmaceutical companies, research institutions, and healthcare providers have accelerated the development and commercialization of CAR T cell therapies. These collaborations facilitate knowledge exchange, resource sharing, and access to patient populations, driving innovation and expanding the product pipelines of key market players.
Challenges:
The CAR T cell therapy market faces challenges related to high treatment costs, complex manufacturing processes, and potential side effects associated with CAR T cell therapy. Overcoming these challenges requires ongoing research, investment in infrastructure, and close monitoring of patient outcomes.
Key Companies:
The Car T Cell Therapy market is dominated by leading companies such as Pfizer, Inc., Novartis AG, Bristol-Myers Squibb, Amgen, Inc., Sorrento Therapeutics, Inc., Johnson & Johnson Services, Inc., Gilead Sciences, Inc., Merck & Co., Inc., and bluebird bio, Inc. Among other players. These companies have established themselves as key players in the market, with strong market presence, extensive research and development capabilities, and a wide range of Car T Cell Therapy products in their portfolios. They employ competitive strategies such as product innovation, strategic partnerships, mergers, and acquisitions to enhance their market share and cater to the diverse needs of patients and healthcare providers.
In June 2022, Bristol-Myers Squibb received FDA approval for Breyanzi (lisocabtagene maraleucel), a CD19-directed chimeric antigen receptor (CAR) T-cell therapy. This approval allows the treatment of adult patients with large B-cell lymphoma (LBCL), further expanding the treatment options available in the market.
Similarly, in April 2022, Kite, a Gilead Company, received FDA approval for Yescarta (axicabtagene ciloleucel) CAR T-cell therapy. This therapy is approved for adult patients with large B-cell lymphoma that is refractory to first-line chemoimmunotherapy or that relapses within 12 months of first-line chemoimmunotherapy.

Need Customized Report for Your Business ?
Utilize the Power of Customized Research Aligned with Your Business Goals
Request for Customized Report- Quick Contact -
- ISO Certified Logo -

















