Bivalent Human Papillomavirus (HPV) Vaccine Market By Product Type (HPV16 Bivalent Vaccine, HPV18 Bivalent Vaccine); By Application (9-16 Years Old, 16-20 Years Old, 20-26 Years Old, 26-45 Years Old); And By Geographic Regions (North America, Europe, Asia Pacific, Latin America, Middle East And Africa) – Global Market Analysis, Trends, Opportunity and Forecast, 2022-2032
Bivalent Human Papillomavirus (HPV) Vaccine Market Size and Overview
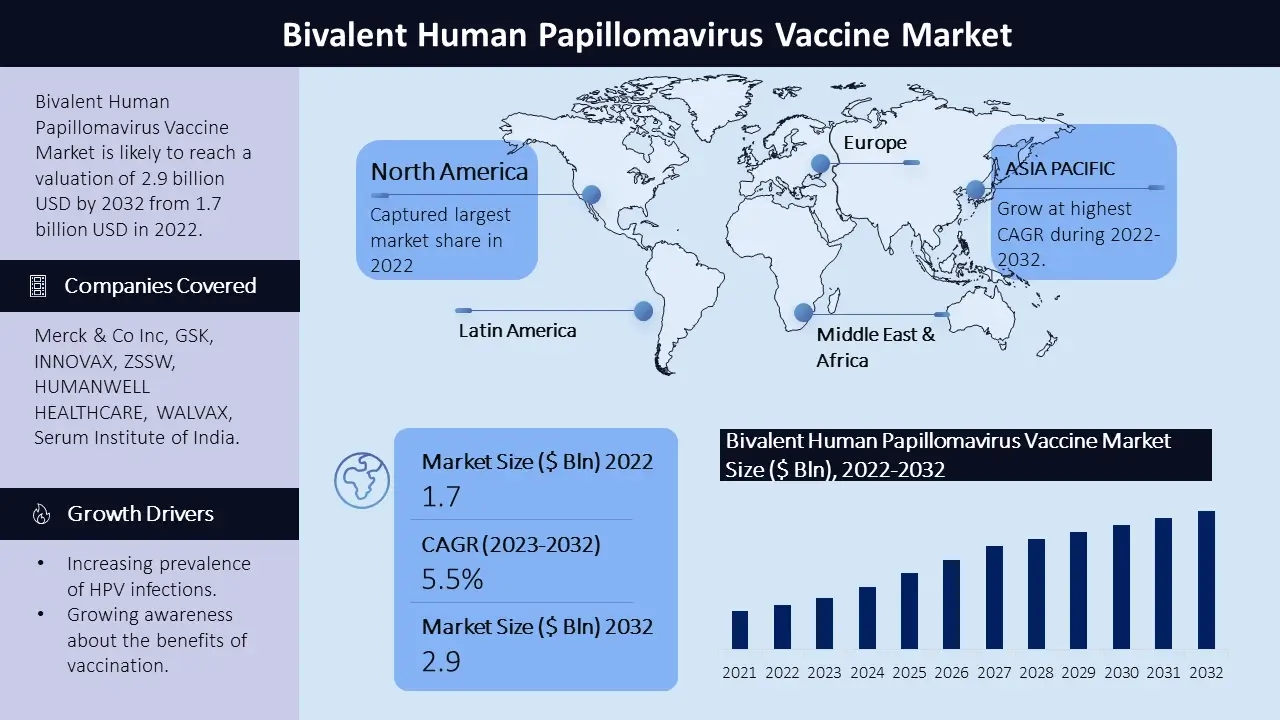
The bivalent human papillomavirus (HPV) vaccine market size is poised to reach USD 2.9 billion by the end of 2032, growing at a CAGR of 5.5% during the forecast period, i.e., 2023 – 2032. In the year 2022, the industry size of bivalent human papillomavirus (HPV) vaccine market was USD 1.7 billion. The reason behind the growth can be attributed to the increasing prevalence of HPV infections, growing awareness about the benefits of vaccination, increasing number of government programs and advancements in vaccine technology. The marketplace incorporates a wide range of products. The market is highly competitive with key players striving to meet evolving customer demands.
Bivalent Human Papillomavirus (HPV) Vaccine Market: Report Scope |
|
|
Base Year Market Size |
2022 |
|
Forecast Year Market Size |
2023-2032 |
|
CAGR Value |
5.5% |
|
Segmentation |
|
|
Challenges |
|
|
Growth Drivers |
|
Bivalent Human Papillomavirus (HPV) Vaccine Market Segmentation
By Product Type
- HPV16 Bivalent Vaccine
- HPV18 Bivalent Vaccine
By Application
- 9-16 Years Old
- 16-20 Years Old
- 20-26 Years Old
- 26-45 Years Old
By Geographic Regions
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
On the basis of Product Type in the bivalent human papillomavirus (HPV) vaccine market the HPV16 bivalent vaccine segment accounted the highest market share in 2022. The HPV16 bivalent vaccine section holds the largest market proportion inside the bivalent HPV vaccine market. This may be attributed to the full-size prevalence of HPV16 infections and the expanded adoption of preventive measures, inclusive of vaccination, to lessen the burden of this precise strain. As HPV16 is one of the excessive-threat traces related to cervical cancer and other HPV-related sicknesses, the HPV16 bivalent vaccine gives effective safety, using its regular increase.
Bivalent Human Papillomavirus (HPV) Vaccine Market Regional Analysis
North America stands as one of the major markets for bivalent human papillomavirus (HPV) vaccine, driven through the well-established healthcare infrastructure. Additionally, emphasis on comprehensive vaccination programs and awareness campaigns targeting HPV infections make contributions to the region’s dominance. The United States, primarily, is a key contributor on this marketplace.
Europe is one of the leading marketplaces for bivalent human papillomavirus (HPV) vaccine because of the region’s increasing focus on preventing HPV-related diseases through vaccination. The European market showcases a developing interest in healthcare solutions, aiming to enhance overall healthcare.
The Asia Pacific bivalent human papillomavirus (HPV) vaccine market is estimated to witness significant growth, during the forecast timeframe led by, increasing healthcare infrastructure, rising awareness about HPV infections. The demand for bivalent human papillomavirus (HPV) vaccine is anticipated to rise in countries like China and India, in which increasing government initiatives to promote vaccination.
Latin America and the Middle East and Africa display steady growth inside the bivalent human papillomavirus (HPV) vaccine market, fueled via awareness campaigns and the integration of vaccination programs into national healthcare systems, increasing focus on preventive healthcare measures in these regions. Key participants to the market's growth in these areas encompass Brazil, Mexico, the United Arab Emirates, and South Africa.
Bivalent Human Papillomavirus (HPV) Vaccine Market Growth Drivers
The increasing prevalence of HPV infections, mainly high-hazard strains associated with cervical cancer and genital warts, has spurred the demand for preventive measures like bivalent HPV vaccination. Cervical cancers persist substantial worldwide fitness concern, and the bivalent HPV vaccine gives an powerful method to reduce its incidence.
Growing awareness about the benefits of vaccination and its role in preventing HPV-associated illnesses has encouraged people and healthcare carriers to actively are searching for vaccination for endangered populations. Government-led vaccination plans concentrated on adolescents, specifically in universities and healthcare centers, have performed a critical position in driving marketplace boom. The growing wide variety of administration programs that aid HPV vaccination has played a pivotal function in riding the adoption and accessibility of bivalent Human Papillomavirus (HPV) vaccines
Advancements in vaccine technology and research have led to the enhancement of improved and more efficient bivalent HPV vaccines. Manufacturers' focus on enhancing vaccine efficacy and safety has garnered greater assurance from healthcare professionals and patients alike.
Bivalent Human Papillomavirus (HPV) Vaccine Market Challenges
challenges in the bivalent HPV vaccine market include the affordability and accessibility of vaccines, particularly in low- and middle-income countries. Ensuring vaccine availability and coverage in remote regions and underserved communities remains a challenge for market players and healthcare organizations.
Bivalent Human Papillomavirus (HPV) Vaccine Market Key Companies
The bivalent human papillomavirus (HPV) vaccine market is poised by several main corporations, each making big contributions to the industry through their sturdy market presence and progressive product offerings. Among these principal players are Merck & Co Inc, Gsk, Innovax, Zssw, Humanwell Healthcare, Walvax, Serum Institute of India, Beijing Minhai Biotechnology Co., Ltd., China National Pharmaceutical Group Corporation (Sinopharm), And Sinovac Biotech Ltd. And other players. These essential players constantly try and revamp their marketplace percentage and meet the desires of a diverse investor base. Their competitive techniques encompass product innovation, forging strategic partnerships, undertaking mergers and acquisitions, and increasing their distribution networks.
In January 2023, Merck & Co Inc. launched its latest bivalent HPV vaccine, Gardasil 9, which provides protection against nine types of HPV, including the two types responsible for cervical cancer.
In May 2022, GSK announced a partnership with the Bill & Melinda Gates Foundation to develop an affordable and accessible bivalent HPV vaccine for people in low- and middle-income countries.

Need Customized Report for Your Business ?
Utilize the Power of Customized Research Aligned with Your Business Goals
Request for Customized Report- Quick Contact -
- ISO Certified Logo -

















